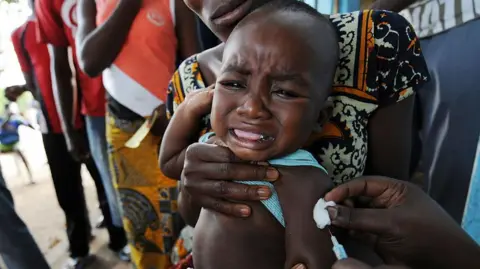
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization as 'unethical'.
The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age. The WHO said it had 'significant concerns' about the plan, describing the birth-dose vaccine as 'an effective and essential public health intervention, with a proven record'.
The US health department, headed by Robert F. Kennedy Jr., who has questioned the effects of vaccines, aimed to use the trial to explore the jab's broader health effects.
The WHO stated that its concerns regarded the study's scientific justification, ethical safeguards, and consistency with established standards for human research. It stressed that the jab had been used for more than three decades in over 115 countries.
By exposing some newborns to a potentially life-saving intervention while denying it to others, the trial could expose them to 'potentially irreversible harm'.
A sizeable portion of Guinea-Bissau’s population is estimated to have hepatitis B, with vaccination at birth preventing virus transmission from mother to baby in 70-95% of cases. The WHO argues that trials allowing one group to receive a placebo are only acceptable when no proven treatment exists, something not applicable to the hepatitis B vaccine.
Despite ongoing efforts to introduce the birth dose nationwide by 2028, the trial involving 14,000 babies funded by the US and led by Danish researchers faced public outrage, leading the Guinea-Bissau government to suspend it. Critics question why newborns in this African country were targeted for such a trial, noting that recently, a US advisory panel stopped recommending the hepatitis B vaccine for all newborns.
The WHO recommends all newborns receive the hepatitis B vaccine within 24 hours of birth to prevent lifelong infection and associated health risks. The ongoing public debate underscores the ethical considerations surrounding global health interventions in vulnerable populations.