Bayer Ltd has recalled a specific batch of Yaz Plus contraceptive pills in South Africa after a mix-up led to incorrectly packaged blister packs containing inactive pills. Women are advised to seek medical advice and return the affected pills for replacement.
Bayer Recalls Yaz Plus Contraceptive Pill in South Africa Due to Packaging Error
Bayer Recalls Yaz Plus Contraceptive Pill in South Africa Due to Packaging Error
A packaging mix-up in a limited batch of Yaz Plus contraceptive pills has resulted in a recall, raising concerns about contraceptive efficacy.
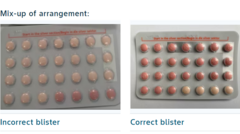
Bayer Ltd has issued a recall for a batch of the popular Yaz Plus contraceptive pill in South Africa due to a significant packaging error that could potentially compromise its contraceptive effectiveness. The situation arose when a number of blister packs from the affected batch, labelled WEW96J and set to expire in March 2026, were incorrectly packaged to contain 24 hormone-free inactive pills instead of the required 24 active hormone pills followed by four inactive ones.
In response to the mix-up, Bayer has urged women to discontinue usage of these pills immediately and to consult their healthcare providers for further guidance. The concern is that users may unknowingly assume they are taking effective hormonal contraception while in reality, they are consuming only inactive pills, which raises the risk of unintended pregnancies.
The South Africa Health Products Regulatory Agency has been informed of the recall, and Bayer is working in close collaboration with health officials to ensure public safety. "While only a limited number of packs from the respective batch is affected, as a precautionary measure, no tablets from these packs shall be used until you have consulted your healthcare practitioner," the recall notice states.
Bayer has taken steps to address the issue, identifying the root cause of the mix-up and implementing corrective measures to prevent similar incidents in the future. The company has set up a dedicated helpline for consumers seeking additional information regarding the recall and replacement options. Anyone in possession of pills from the affected batch is encouraged to return them to pharmacies for a refund or a replacement.
This incident highlights the importance of vigilance in pharmaceutical packaging and the potential risks that can arise from mistakes in this critical industry. Bayer has assured the public that this issue is isolated to one batch and no other products are affected, reinforcing their commitment to consumer safety and transparency.


















