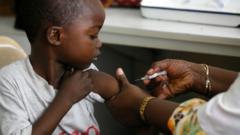
The long-fought battle against malaria has reached a pivotal moment with the recent approval of the first medication specifically designed for babies and very young children. This groundbreaking drug is expected to be distributed across African nations within a matter of weeks, a vital development in light of the ongoing malaria crisis that claims hundreds of thousands of lives annually.
Currently, malaria is responsible for nearly 597,000 deaths each year, predominantly impacting Africa where the majority of victims are children under five. Until now, treatment regimens for infants often relied on formulations created for older children, creating significant risks of overdose and side effects due to the difference in liver development and drug processing in very young bodies.
The newly approved drug, branded Coartem Baby or Riamet Baby in various regions, has been developed by Novartis in collaboration with the Medicines for Malaria Venture (MMV), a not-for-profit organization backed by international supporters including the UK, Swiss, and Dutch governments, along with the World Bank and the Rockefeller Foundation. This partnership aims to sustain its efforts on a largely not-for-profit basis, thereby improving accessibility for the most vulnerable populations.
Vas Narasimhan, CEO of Novartis, highlighted the importance of this milestone in the fight against malaria. "For more than three decades, we have stayed the course in the fight against malaria, working relentlessly to deliver scientific breakthroughs where they are needed most." He emphasized the company’s commitment to ensuring even the smallest and most vulnerable patients receive proper care.
In the developer's view, this advancement is pivotal in addressing the existing treatment gap, as most medications inadequately target the needs of infants, leaving them susceptible to severe health risks. According to Martin Fitchet, CEO of MMV, the introduction of Coartem Baby is a critical step toward alleviating the devastating burden malaria imposes particularly on infants and children. "But with the right resources and focus, it can be eliminated," he stated, recognizing the potential impact of this new treatment.
Dr. Marvelle Brown, an associate professor from the University of Hertfordshire, calls this approval a monumental breakthrough that could drastically reduce malaria-related mortality rates among infants in sub-Saharan Africa, where over 76% of malaria deaths occur in young children.
The approval of Coartem Baby marks an essential addition to the global antimalarial arsenal, and experts are hopeful that with its introduction, healthcare inequalities may be reduced, offering a lifeline to the most at-risk populations.